Abstract
Introduction:The ongoing German Antihemophilic factor (recombinant) (rAHF) Hemophilia A (HA) outcome Database (AHEAD) study (DRKS00000556) evaluates the real-world, long-term effectiveness and safety of rAHF (Advate®; Baxalta US Inc., a Takeda company, Lexington, MA, USA) and rurioctocog alfa pegol (Adynovi®; Baxalta US Inc., a Takeda company, Lexington, MA, USA) in patients with HA treated in routine clinical practice. The objective of this analysis was to describe the hemostatic effectiveness of rurioctocog alfa pegol administered as standard prophylaxis (SP) and individualized pharmacokinetic-guided prophylaxis (PKP) in adult patients (≥18 years of age) with severe HA (factor VIII activity ˂1%).
Methods: The German AHEAD study is a non-interventional, prospective, multicenter study. This analysis focused on patients switched from rAHF to rurioctocog alfa pegol or enrolled on rurioctocog alfa pegol during 7 years of follow-up (data cutoff: Jun 30, 2020), using a time-free method of analysis to provide average values for each outcome per patient per treatment year, irrespective of the duration of study follow-up. Key outcomes included annualized bleeding rates (ABRs [all bleeds]), annualized joint bleeding rates (AJBRs), factor consumption, and factor VIII trough levels expressed in patient-years. Adverse events were also reported. In the analyses presented for bleeding rates and consumption, the switch year was excluded. These results focus on rurioctocog alfa pegol; however, outcomes for rAHF are included in the table for comparison. This study was reviewed/approved by the relevant institutional review boards/ethics committees of all participating centers.
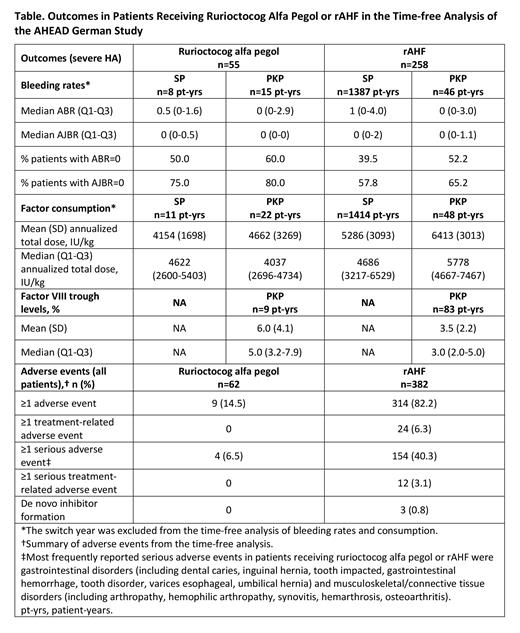
Results: A total of 382 patients were receiving rAHF at baseline; 60 of these switched from rAHF to rurioctocog alfa pegol during the study. Two additional patients were enrolled on rurioctocog alfa pegol, giving a total of 62 patients in the rurioctocog alfa pegol group (severe HA, n=55; moderate HA, n=7; PKP, n=27; SP, n=35). Median age (range) for the 322 patients who received rAHF throughout the study (severe HA, n=258; moderate HA, n=64) was 24 (1-80) years. In the time-free analysis of bleeding rates, the combined rurioctocog alfa pegol data for enrolled patients and switched patients with severe HA resulted in a total of 23 patient-years of data for rurioctocog alfa pegol (SP, n=8 patient-years; PKP, n=15 patient-years). Median (Q1-Q3) ABRs were lower in patients receiving PKP with rurioctocog alfa pegol vs SP (0 [0-2.9] vs 0.5 [0-1.6]). Similar median AJBRs were observed between the PKP and SP groups (Table). A higher proportion of patients receiving PKP vs SP had an ABR or AJBR of zero (ABRs, 60% vs 50%; AJBRs, 80% vs 75%, respectively). Mean annualized total dose for rurioctocog alfa pegol was 4154 IU/kg for SP and 4662 IU/kg for PKP. Mean (median [Q1-Q3]) target FVIII trough levels for patients with severe HA receiving rurioctocog alfa pegol PKP were 6.0% (5.0% [3.2%-7.9%]). For patients receiving rAHF PKP, the mean (median [Q1-Q3]) target trough levels were 3.5% (3.0% [2.0%-5.0%]). Adverse events occurred in 9/62 (14.5%) patients treated with rurioctocog alfa pegol in the time-free analysis; 4 (6.5%) were serious adverse events and none were treatment related. De novo inhibitors were reported for 3 patients in the rAHF group and for none in the rurioctocog alfa pegol group.
Conclusion: These real-world data showed lower bleeding rates with rurioctocog alfa pegol PKP versus SP in the time-free analysis.
Klamroth: Daiichi Sankyo: Other, Speakers Bureau; LEO: Other, Research Funding, Speakers Bureau; Uniqure: Consultancy, Other; Sobi: Consultancy, Other, Speakers Bureau; Sanofi: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Octapharma: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Roche/Cugai: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Biotest: Consultancy, Other: Travel support, Speakers Bureau; BioMarin: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Shire (a Takeda company): Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Pfizer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Bayer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Grifols: Speakers Bureau. Kurnik: Bayer: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Research Funding, Speakers Bureau; Pfizer: Research Funding; Roche: Consultancy, Research Funding, Speakers Bureau; Shire (a Takeda company): Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Research Funding; Biotest: Consultancy, Speakers Bureau. Wenning: Bayer: Research Funding; Biotest: Consultancy, Research Funding; CSL Behring: Research Funding; Octapharma: Research Funding; Shire (a Takeda company): Consultancy, Research Funding; Sobi: Consultancy, Research Funding; Roche: Consultancy, Research Funding, Speakers Bureau. Escuriola-Ettingshausen: Biotest: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Research Funding, Speakers Bureau; Octapharma: Consultancy, Research Funding, Speakers Bureau; Shire (a Takeda company): Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Research Funding, Speakers Bureau; BioMarin: Consultancy; Grifols: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Roche/Chugai: Consultancy, Speakers Bureau; Bayer: Speakers Bureau; Kedrion: Speakers Bureau; Pfizer: Speakers Bureau. Regensburger: Takeda Pharma Vertrieb GmbH & Co. KG: Current Employment. Tang: Takeda Pharmaceuticals International AG: Current Employment; Takeda: Current equity holder in publicly-traded company. Gu: Takeda Development Center Americas, Inc.: Current Employment; Takeda: Current equity holder in publicly-traded company. Guerra: Takeda Pharmaceuticals: Current Employment; Takeda: Current equity holder in publicly-traded company. Oldenburg: Novo Nordisk: Consultancy, Honoraria, Research Funding, Speakers Bureau; Freeline: Consultancy, Honoraria, Speakers Bureau; Grifols: Consultancy, Honoraria, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding, Speakers Bureau; CSL-Behring: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; Biotest: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Speakers Bureau; University Clinic Bonn AöR: Current Employment; Octapharma: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Shire (a Takeda company): Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal